Overview
Device design: a complex multi-disciplinary venture
The regulation of device development is critical to ensure that patients have timely access to innovative medical device technologies whilst protecting device quality, performance and safety standards.
The variation in complexity, risk profile and application of medical devices makes the implementation and enforcement of harmonised regulation and business processes a challenging, but none the less, important task.
Medical device innovation requires the developer to be au-fait with many interrelated concepts and requirements. Medtech Translate has been designed to meet this need in that it provides centralised guidance and information and practical support to its members on key concepts including the regulatory requirements associated with bringing a medical device to market.
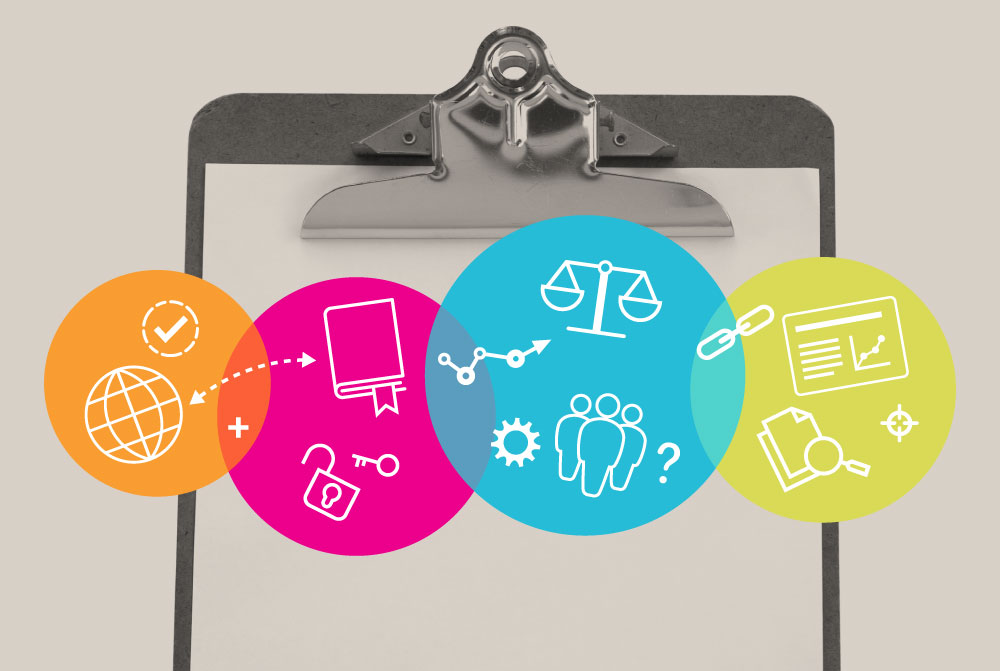

Medtech Translate membership and benefits
MedTech Translate, designed to assist its members in understanding key concepts and requirements associated with bringing a new medical device to market, also hosts a clinical advisory support function whereby members can request input, advice or support for their device development project from other MedTech Translate members with clinical research translation or trials experience.
MedTech Translate membership is free and open to anyone with an active interest in medical device development or research translation.
There are three categories of membership:
- Standard – provides full access to Medtech Translate information and resources
- Clinical advisory (CV required) – standard plus device development support programme sign up
- Clinical advisory+ (CV required) – as 2 above with linkage to a clinical trials feasibility programme
Device development is a journey
Medtech Translate describes the device development process as a staged journey. Navigation is needs driven allowing users to bring together content thematically, by topic or in a ‘stage-of-development’ focused manner – search outputs will be tailored to reflect the users project status from initiation, feasibility, verification/validation right through to launch.
In addition to its guidance and information role Medtech Translate is also designed to support access to a ‘clinical expertise / advisory’ registry where members can request advice or support pertinent to their project needs from Medtech Translate members with a clinical background and an expressed an interest in, or experience of, supporting medical device development.
The device development journey, as described by Medtech Translate, builds on an existing device development model which describes the product realisation process in five discrete stages interspersed with a set of critical ‘go-no-go’ decision gates at the end of each stage. This stage-gated model describes the activities performed by each function (e.g. regulatory, clinical, quality) of the business as well as the expected deliverables and key considerations to be addressed at each stage. Ref: Pietzsch et al., 2009. DOI: 10.1115/1.3148836.
Click on the icons for a description of each stage

Stage 1
Initiation

Stage 2
Formulation

Stage 3
Design &
Development

Stage 4
Final Validation
Stage 5
Post-Market
Medtech Translate Origins
CRDI CÚRAM Partnership


The Medtech Translate knowledge portal, in supporting the progression of device research and development, is an important output of the Clinical Research Development Ireland (CRDI) / CÚRAM collaborative partnership. CRDI is a funded partner in CÚRAM, SFI Research Centre for Medical Devices. CÚRAM’s researchers focus on designing and manufacturing ‘smart’ medical devices to improve health outcomes and quality of life for those with chronic illness.
The partnership focuses on the development of methods and media to support excellence in medical device research to include raising awareness of the potential of regulation to impact the successful translation of medical device research. Work focuses on capturing clinical medical device development experience and the provision of training and information resources with a view to promoting the incorporation of ‘principles of best practice’ from the earliest stages of research right through all stages of the device development process.
Stakeholder engagement
The core objectives of the CRDI CÚRAM partnership as well as the development and delivery of its initiatives were underpinned by the support and advice of industry, regulatory and clinical trials experts. The partnership would, in particular, like to acknowledge the significant contribution made by the National Standards Authority of Ireland, the Health Products Regulatory Authority, Aerogen Ltd and HRB Clinical Research Coordination Ireland to the realisation of partnership ambitions.



Medtech Translate has emanated from research conducted with the financial support of Science Foundation Ireland (SFI) and the European Regional Development Fund (ERDF) under grant number 13/RC/2073.


